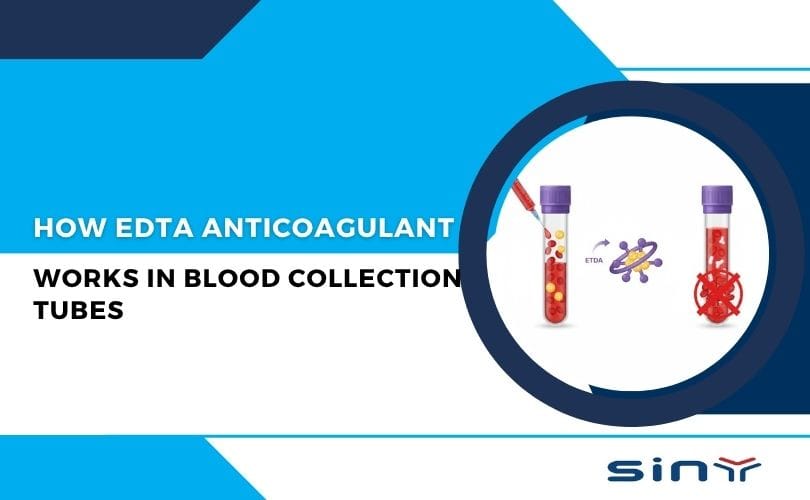
When a blood sample is drawn, the natural tendency of blood is to clot—a crucial biological process that prevents excessive bleeding. However, in medical testing, clotting can destroy the sample’s integrity, making it useless for analysis. That’s where EDTA anticoagulant steps in.
EDTA works by binding calcium ions (Ca²⁺)—an essential component required for the clotting process. By chelating calcium, EDTA prevents clot formation and keeps the blood in a liquid state, making it ideal for laboratory tests.
You can learn more about specialized EDTA blood collection tubes at EDTA Tube Official Website.
What is EDTA Anticoagulant?
EDTA, short for Ethylenediaminetetraacetic acid, is a chelating agent—a molecule that forms stable complexes with metal ions like calcium and magnesium.
In blood collection, EDTA is used in two common salt forms:
K₂EDTA (Dipotassium EDTA)
K₃EDTA (Tripotassium EDTA)
These salts are added to blood collection tubes—often referred to as EDTA tubes—to prevent clotting. The tubes are typically identified by their lavender or purple caps in clinical laboratories.
For more about the types of EDTA tubes, check out EDTA Tubes for Blood Collection.
How Does EDTA Anticoagulant Work?
The mechanism of EDTA anticoagulant is both simple and ingenious. When blood is drawn into an EDTA tube, the EDTA molecules immediately begin to bind with free calcium ions in the blood plasma. Calcium is a critical cofactor in the coagulation cascade, a series of enzymatic reactions that lead to blood clot formation. By removing calcium ions, EDTA effectively halts this cascade, preventing the blood from clotting.
This process ensures that blood samples remain in a liquid state, preserving the cellular components and allowing for accurate analysis. For a deeper dive into the principle of EDTA anticoagulant, visit this detailed explanation.
Mechanism of Anticoagulation
- Chelating Agent: EDTA is a powerful synthetic molecule classified as an aminopolycarboxylic acid and a chelating agent.2
- Targeting Calcium (3$Ca^{2+}$):4 EDTA has an extremely high affinity for divalent metal ions, most importantly calcium ions (5$Ca^{2+}$), which are essential cofactors in multiple steps of the blood clotting process (the coagulation cascade).6
- Forming a Stable Complex: When blood is drawn into the purple-top tube and mixed, the EDTA rapidly binds to the free calcium ions, forming a highly stable, soluble ring-like structure called a chelate (7$EDTA-Ca^{2+}$ complex).8
- Inhibition of Clotting: By sequestering and “locking up” the functional calcium ions, EDTA makes them unavailable to the clotting factors (enzymes).9 Without the calcium cofactors, the coagulation cascade cannot proceed to form fibrin, and thus, the blood cannot clot.10
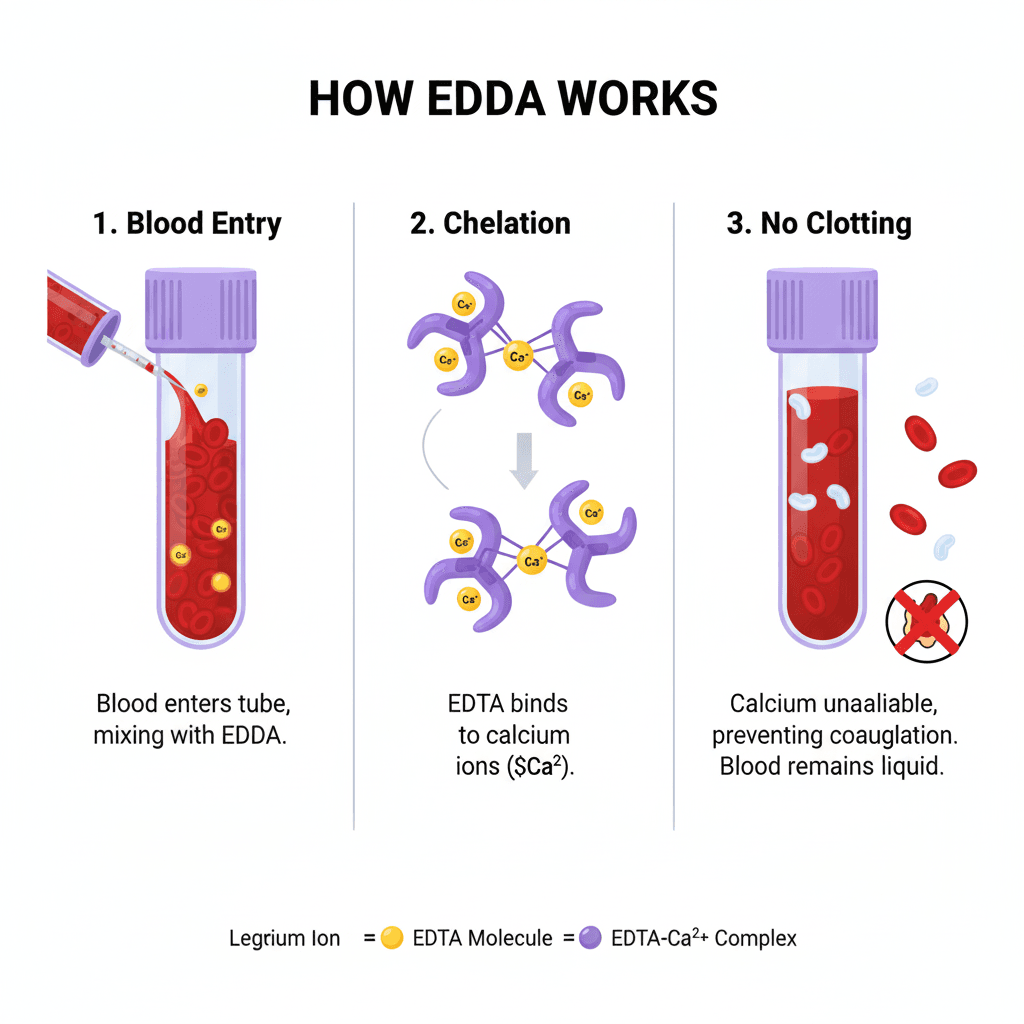
The outcome? A stable, non-clotted blood sample that’s ideal for hematology, molecular diagnostics, and blood cell counting.
You can dive deeper into the chemical process by visiting What is the Principle of EDTA Anticoagulant?.
Chemical Properties of EDTA
EDTA’s efficiency lies in its molecular structure—it has four carboxylic acid groups and two amine groups, enabling it to bind up to six metal ions simultaneously.
| Property | Details |
|---|---|
| Chemical Name | Ethylenediaminetetraacetic acid |
| Chemical Formula | C₁₀H₁₆N₂O₈ |
| Molecular Weight | 292.24 g/mol |
| pH Range in Tubes | 7.0 – 8.0 |
| Chelation Ions | Ca²⁺, Mg²⁺, Fe³⁺ |
Its strong chelating ability and chemical stability make it the most preferred anticoagulant for hematological use.
Why EDTA is Used in Blood Collection Tubes
There are several reasons why EDTA is the gold standard for anticoagulation in clinical laboratories:
Preserves blood cell morphology: Unlike heparin or citrate, EDTA maintains the natural shape and size of blood cells, ensuring accurate cell counts and differential tests.
Prevents platelet clumping: This ensures reliable platelet count measurements.
Stable for longer durations: Blood samples remain viable for hours, allowing flexibility in testing.
Compatible with automated analyzers: EDTA-treated blood samples produce precise results on modern hematology machines.
For professional-grade EDTA tubes, explore Siny Medical’s EDTA Tubes and their manufacturing standards.
Applications of EDTA Anticoagulant in Blood Collection
EDTA anticoagulant is widely used in medical laboratories for a variety of tests. Here are some of its key applications:
- Complete Blood Count (CBC): EDTA preserves the morphology of blood cells, making it ideal for CBC tests, which analyze red blood cells, white blood cells, and platelets.
- Blood Typing: EDTA tubes are used to collect blood samples for blood group and Rh factor determination.
- Molecular Diagnostics: EDTA is preferred for DNA and RNA extraction due to its ability to inhibit nucleases.
- Flow Cytometry: EDTA ensures the stability of cell surface markers, which is crucial for flow cytometry analysis.
For more information on the applications of EDTA tubes, check out this resource.
How EDTA Compares to Other Anticoagulants
There are several anticoagulants used in laboratory testing—each with its unique mechanism and application.
Here’s how EDTA stacks up against the competition:
| Anticoagulant | Mechanism | Best Used For | Limitations |
|---|---|---|---|
| EDTA | Chelates calcium ions | Hematology tests, CBC | Not suitable for coagulation studies |
| Heparin | Inhibits thrombin | Biochemistry, plasma assays | Alters WBC morphology |
| Sodium Citrate | Binds calcium reversibly | Coagulation studies | Short sample stability |
| Oxalate/Fluoride | Precipitates calcium | Glucose estimation | Can damage blood cells |
For a detailed discussion, visit EDTA vs Other Anticoagulants.
Top Quality Control Tips for Using EDTA Tubes
To ensure accurate results, laboratories must follow strict quality control measures when handling EDTA blood samples.
Here are some expert recommendations inspired by Top Quality Control Tips for Using EDTA Tubes:
Proper mixing: Immediately after blood collection, gently invert the tube 8–10 times. Avoid shaking vigorously.
Fill volume accuracy: Always fill the tube to the recommended mark to maintain the correct blood-to-anticoagulant ratio.
Avoid hemolysis: Use a smooth venipuncture technique to prevent red blood cell damage.
Storage: Keep samples at room temperature and process within 4–6 hours for optimal accuracy.
These simple yet crucial practices maintain the reliability of test results and ensure smooth operation in clinical laboratories.
Safety and Handling Guidelines
Although EDTA is safe in the small quantities used in blood collection, certain safety measures should always be followed:
Wear protective gloves when handling blood samples.
Dispose of used tubes in biohazard containers.
Avoid contamination by using sterile needles and tubes.
Manufacturers like EDTA Tube and Siny Medical maintain stringent safety and quality standards during production, ensuring medical-grade reliability.
EDTA Separation Gel Coagulation Blood Collection Tube
Some EDTA tubes are equipped with a separation gel that facilitates the separation of plasma from blood cells during centrifugation. This innovation enhances the efficiency of sample processing and reduces the risk of contamination. To learn more about this advanced tube design, visit EDTA Separation Gel Coagulation Blood Collection Tube.
Summary
EDTA anticoagulant is a cornerstone of modern blood collection, offering unparalleled benefits in preserving sample integrity and ensuring accurate test results. Its ability to bind calcium ions prevents clotting while maintaining cell morphology, making it indispensable for hematological and molecular diagnostics. By following best practices and understanding its applications, medical professionals can maximize the effectiveness of EDTA tubes.
For more information on EDTA tubes and their applications, visit EDTA Tube.
This blog is proudly presented by EDTA Tube, your trusted source for high-quality blood collection tubes. Explore our range of products and resources at About Us and feel free to Contact Us for any inquiries.
For additional insights, watch our informative videos on YouTube or explore our products on Made-in-China.
FAQs
1. What is the main function of EDTA in blood collection?
EDTA’s main role is to prevent blood from clotting by chelating calcium ions that are essential for the coagulation process.
2. Why are EDTA tubes purple?
The purple cap color helps lab technicians easily identify that the tube contains an EDTA anticoagulant.
3. Can EDTA affect biochemical tests?
Yes. Since EDTA binds metal ions like calcium and magnesium, it can interfere with biochemical tests requiring these elements.
4. What is the shelf life of EDTA tubes?
Most EDTA tubes have a shelf life of 18–24 months when stored under recommended conditions.
5. How much EDTA is typically used in a blood tube?
Usually, around 1.5–2.0 mg of EDTA per mL of blood ensures optimal anticoagulation.